Human-centric design and evaluation

The integration of Human Factors in the design, development, implementation and evaluation of Healthcare Technologies is a vital part of ensuring that:
We incorporate Human Factors expertise into the device design across the membership of the Centre for Healthcare Technologies, to achieve an evidenced based, human-centred approach to the research into and development of novel healthcare technologies.
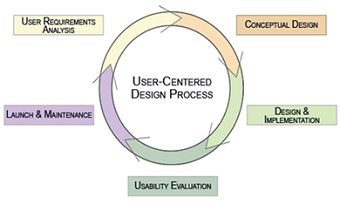
User-centred design process
The user-centred design process features incorporates the following features in a continual feedback loop:
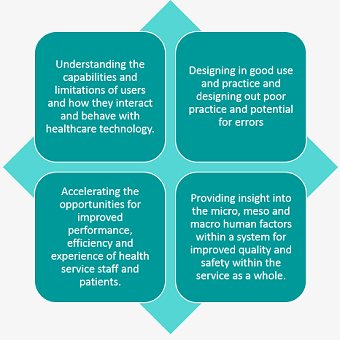
Why incorporate human factors into medical device design?
Some of the benefits to developers of healthcare technologies, health service providers and procurement pathways from integrating Human Factors into their processes are shown below:
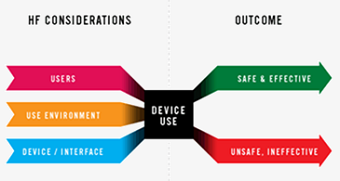
There is an increasing demand for the integration of HFE (Human Factors and Ergonomics) not only in healthcare technology design but also within health service provision, systems and organisation. The Human Factors Research Group (HFRG) can provide a comprehensive service of HFE methods and tools to understand what is required of novel healthcare technologies by all users, how they will be used and integrated more widely into the health service and / or community care.
Expertise offered by HFRG:
-
User Requirements Capture
-
User Centred Design
-
Analysis of the sociotechnical systems in which technologies will need to operate
-
Usability Testing
-
Workload Analysis (cognitive and physical)
-
Error and Reliability Analysis
-
Development of training, education and documentation for technology use
-
User Experience
Experts
Sarah Atkinson
Holly Blake
Sue Cobb
Alexandra Lang
Sarah Sharples
Related Centres, Institutes and Groups
Human Factors Research Group
MindTech
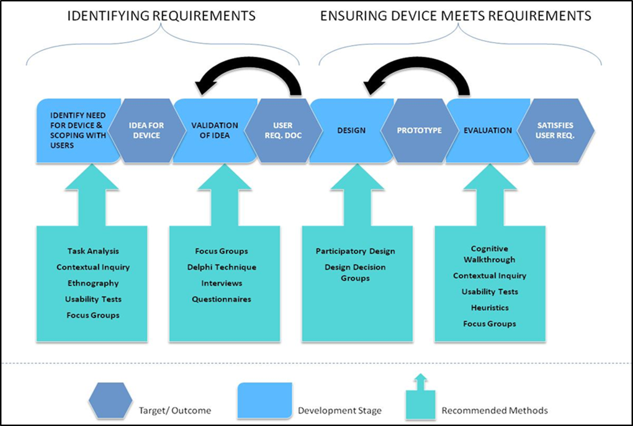
The Product Development Lifecycle

The HFRG (Human Factors Research Group) team has significant experience of working with developers of technology from early requirements capture to concept generation, iterative development and usability testing through to formative and summative evaluation stages.
Our research and applied work
-
Uses Human Factors to understand user requirements of health technologies and medical devices
-
Involves users in the development of our methods and research design
-
Utilises user-centred and participatory approaches with users and stakeholders throughout the design and evaluation of medical and healthcare technologies
-
Entails the expert application of a wide range of methods at the appropriate time during a design lifecycle
Exemplar projects
-
Design, Development and Evaluation of digital games in the field of hearing aid technologies – 3D Tunein (H Patel, M Hallewell, M D’Cruz, R Eastgate, S Cobb)
-
Vision Normality from Virtual Unreality - Interactive Binocular Therapy (I-BiT™) for treatment of lazy eye (amblyopia) (R Eastgate, S Cobb)
-
System Evaluation - Using cognitive work analysis to explore the role of mobile technologies in the anaesthetists work practice (A Salazar Barrera, A Lang, S Sharples)
-
System Evaluation - Staff experience of Technology intervention, EObs and Handheld Technologies in the Ward (A Lang, J Pinchin, S Sharples)
Working with different stakeholders in health technology development and evaluation

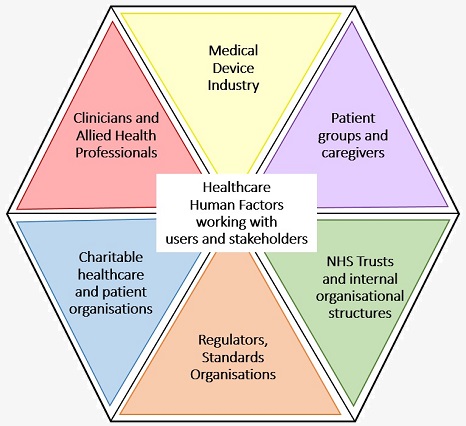
HFRG (Human Factors Research Group) work with a wide range of users, involving them in our research methods as potential users or proxy users of devices and systems. They are involved throughout the technology development processes and also play a key role in stakeholder consultations when considering the organisational or cultural impacts of a novel intervention or system/ process change.
As examples of our participatory methods, we have involved the following user groups and stakeholders in our projects:
An underlying principle of Human Factors is ‘fitting the task to the human’ and as such we integrate this ethos throughout our research planning and design as well as the objectives of research enquiries. In doing this we can ensure that the needs of users and stakeholders are met throughout their interaction with research projects aswell as in the devices and systems that we help to design and evaluate.
Exemplar projects
-
User Requirements Capture and Evaluation with Industrial Partners (A Lang, J Martin, S Sharples)
-
Medical Device Design for Adolescents (A Lang)
-
Product Design for Older Users HF collaboration with elderly users and development teams (S Cobb, A Floyd, R Edlin-White)
-
Design and development of a technology system for teenage health promotion and awareness– PEGASO: Personalised Guidance Services for Optimising Lifestyle in Teenagers (A Lang, S Atkinson, S Cobb)