Preventing infections - Antimicrobial devices and coatings
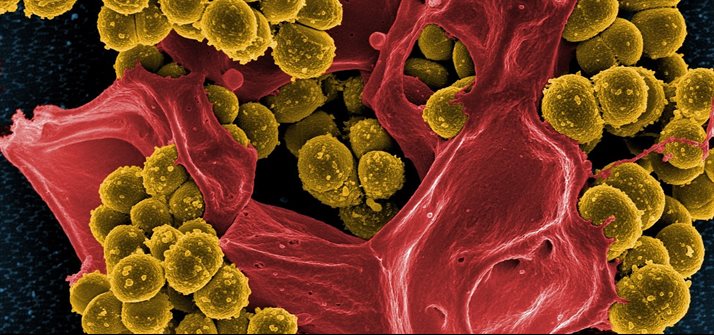
The emergence and spread of microbes resistant to antibiotics is one of the greatest global threats to health. Surgical devices and implantable devices often introduce infections to the body and we are exploring different ways to prevent these, making the devices antimicrobial, with commercial success.
Bacterial-resistant coatings
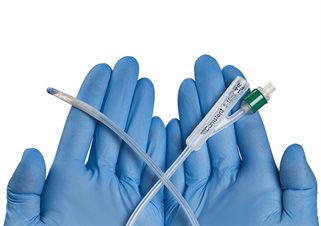
We have developed a patented class of materials which are resistant to bacterial attachment. Used as a medical device coating, the materials resist bacterial attachment, rather than killing the bacteria. This has the advantage that that biofilm colonisation is prevented, potentially preventing infection in patients, and that the coating should therefore not contribute to bacterial resistance.
Camstent urinary catheters
This technology has been commercialised for urinary catheters in collaboration with Camstent.
More about the bacterial-resistant technology and its commercialisation journey with Camstent
Antimicrobial devices
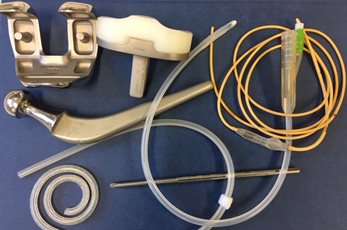
We have had great success in making the devices themselves antimicrobial. By impregnating the device materials with antimicrobials the antimicrobials continually migrate to the surface of the device, replacing surface-coated materials and allowing the antimicrobial capability to last for up to 100 days - vital for in-dwelling devices.
Codman hydrocephalus shunts
This technology was commercialised by Codman for hydrocephalus shunts, and has been reducing infections worldwide since 2003 for over a million patients.
More about the antimicrobial impregnated devices and and about the latest application to peritoneal catheters